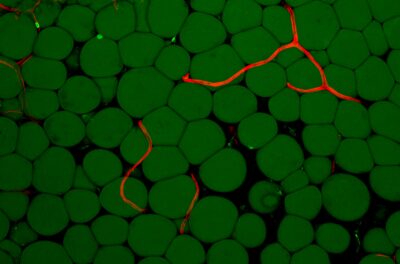
We study vessels, their organ-specific properties and responses to diseases.
Blood vessels wire the entire body and act as highways for fast and efficient transport of nutrients, hormones, cells, and waste products between tissues. Depending on the organ they reside in, blood vessels show different characteristics and perform various functions. For instance, sinusoidal blood vessels, which have larger gaps between endothelial cells, allow the passage large macromolecules and help the liver to carry out its filtration function. On the other hand, the specialized capillaries of the brain form a tight barrier to strictly regulate what can cross into the brain tissue. How the vessels of different organs acquire and maintain such varying phenotypes and functions is still a topic for further investigation.
Our current research focus is to understand the pathological changes that occur in the endothelial cells of various organs in metabolic diseases. Both obesity and diabetes are strongly linked to cardiovascular diseases and endothelial cell dysfunction. However, the mechanisms by which these diseases affect organ-specific vessels are not yet fully known.
We aim to uncover these mechanisms by using mouse models of metabolic diseases and patient samples, followed by molecular analyses of the isolated endothelial cells and their interactions with the stromal and parenchymal cells. The results from these studies will allow us to understand the pathological processes that lead to disease progression. Such deeper understanding is of utmost importance in the development of novel modes of prevention and treatment of pathological changes and in improvement of patient health by reducing cardiovascular complications of obesity and diabetes.
Obesity and overweight related comorbidities have been estimated to cost the European Union over 60 billion euros annually through healthcare costs and lost productivity. The energy surplus in obesity results in adipocyte hypertrophy and a rapid expansion of adipose tissue, which cannot be matched by the growth of new blood vessels. The resultant chronic inflammation, adipocyte death, hypoxia, and a blunted response to insulin (insulin resistance) lead to a decreased capacity of adipocytes to store lipids. This leads to elevated free fatty acid levels in the blood circulation, which contributes to the development of other metabolic diseases, such as non-alcoholic fatty-liver disease, which is the most frequent liver disease worldwide. In fact, it has been suggested that healthy expansion of adipose tissue and its proper vascularization could be protective against ectopic fat accumulation in obesity-related diseases.
Most metabolic diseases are accompanied by endothelial cell dysfunction and a loss of microvasculature, termed “capillary rarefaction”. Due to the organ-specific features and functions of different capillary networks, obesity-induced capillary rarefaction may contribute to various systemic consequences, depending on the affected tissue. For instance, loss of capillaries from skeletal muscle may impact glucose homeostasis, whereas in pancreatic islets, capillary loss can cause insufficient insulin secretion to the blood circulation. In the case of adipose tissue, capillary rarefaction results in reduced tissue perfusion, tissue oxygenation, and insulin delivery to the adipocytes, impeding adipose tissue function. Thus, normalization of the adipose tissue vasculature can enable proper delivery of insulin and lipids to adipocytes and improve adipose tissue function, reducing ectopic lipid accumulation in the liver and skeletal muscle.
Endothelial dysfunction and capillary rarefaction have been observed in adipose tissue, skeletal muscle, heart, kidney, and brain. However, surprisingly little is known about the molecular mechanisms that control these phenotypes in different organs. We aim to shed more light ontoon these mechanisms by looking at the changes that occur in endothelial cells of various organs in metabolic diseases using a multi-omics approach. We also combine our findings from mouse disease models with human data, to find common pathways in both species, so these pathological pathways are relevant and can be experimentally studied in mouse models. Our results can help reveal how such organ-specificity is governed, whether the organ-specificity is due to the inherent characteristics of the endothelial cells or due to the microenvironment, and whether such particular organ-specific features can be induced in other tissues and whether the organ-specificity is due to the inherent characteristics of the endothelial cells or due to the microenvironment provided by the tissue parenchyma.
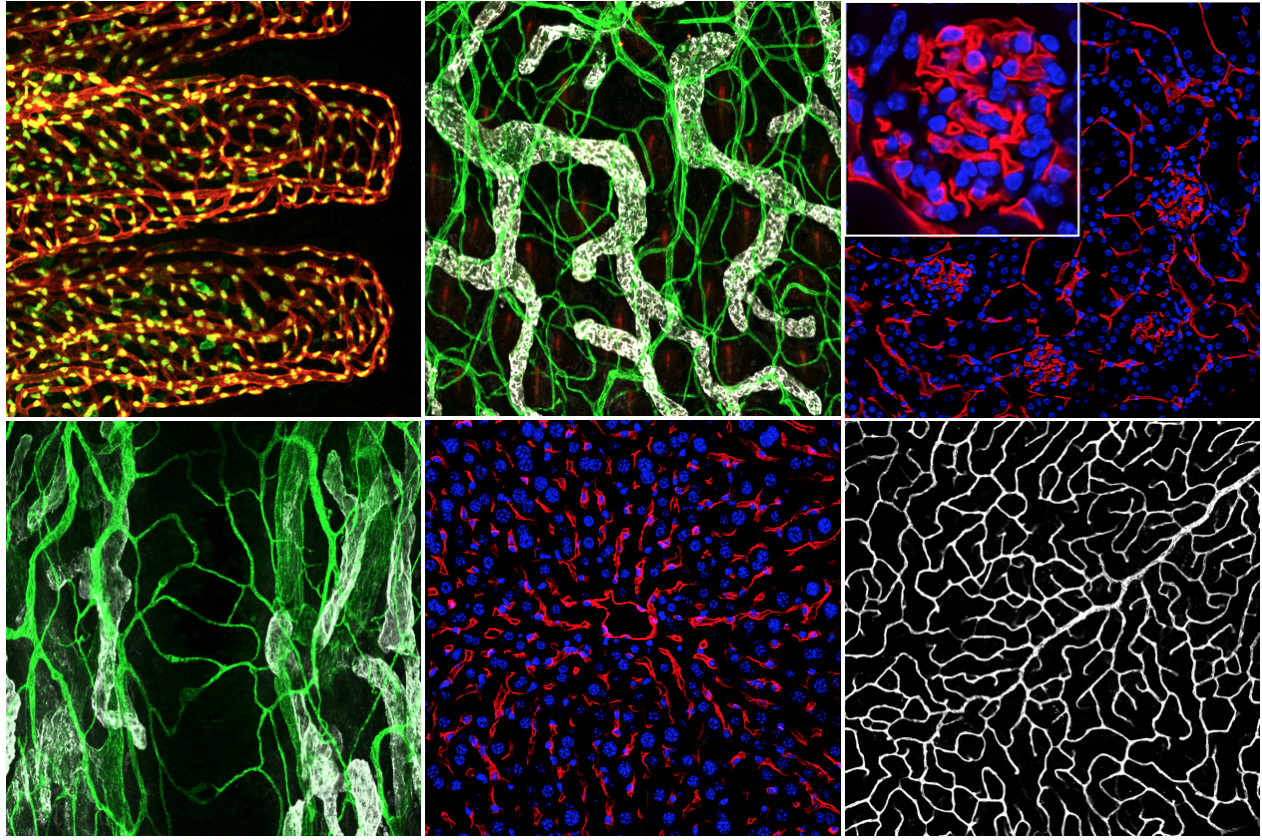
A closer look at adipose tissue lymphatics and their markers. Lackman MH, Subashi Y, Karaman S. Curr Opin Hematol. 2022 Feb 25
Interplay of vascular endothelial growth factor receptors in organ-specific vessel maintenance. Karaman S, Paavonsalo S, Heinolainen K, Lackman MH, Ranta A, Hemanthakumar KA, Kubota Y, Alitalo K. J Exp Med. 2022 Mar 7;219(3):e20210565
Capillary Rarefaction in Obesity and Metabolic Diseases-Organ-Specificity and Possible Mechanisms. Paavonsalo S, Hariharan S, Lackman MH, Karaman S. Cells. 2020 Dec 14;9(12):E2683.
Vascular endothelial growth factor signaling in development and disease. Karaman S, Leppänen VM, Alitalo K. Development. 20 Jul 2018;145(14). pii: dev151019
Development and plasticity of meningeal lymphatic vessels. Antila S, Karaman S, Nurmi H, Airavaara M, Voutilainen M, Mathivet T, Chilov D, Li Z, Koppinen T, Park JH, Fang S, Aspelund A, Saarma M, Eichmann A, Thomas JL, Alitalo K. J Exp Med. 15 Nov 2017. pii: jem.20170391
VEGFR3 modulates vascular permeability by controlling VEGF/VEGFR2 signaling. Heinolainen K, Karaman S, D’Amico G, Tammela T, Sormunen R, Eklund L, Alitalo K, Zarkada G. Circ Res. 28 Apr 2017;120(9):1414-1425.
Transgenic overexpression of VEGF-C induces weight gain and insulin resistance in mice. Karaman S, Hollmén M, Yoon SY, Alkan HF, Alitalo K, Wolfrum C, Detmar M. Sci Rep. 11 Aug 2016;6:31566
Blockade of VEGF-C and VEGF-D modulates adipose tissue inflammation and improves metabolic parameters under high-fat diet. Karaman S, Hollmén M, Robciuc MR, Alitalo A, Nurmi H, Morf B, Buschle D, Alkan HF, Ochsenbein AM, Alitalo K, Wolfrum C, Detmar M. Mol Metab. 4 Dec 2014;4(2):93-105.
CURRENT GROUP MEMBERS
Emmi Pakarinen, Postdoctoral fellow
Satu Paavonsalo, Doctoral student
Yelin Subashi, Doctoral student
Madeleine Lackman, Doctoral student
Alexandra Mäkelä, Master’s student
Selvin Yildiz, Master’s student
FORMER GROUP MEMBERS
Mari Jokinen (Bioanalyst / research technician)
Sangeetha Hariharan (Doctoral student)
Akseli Bonsdorff (Medical student)
Sonja Leppänen (Undergraduate student)
Noora Vilander (Technical trainee)
Amanda Ranta (Undergraduate student, completed BSc thesis in the group)