We have a long-standing interest in studying mechanisms of organ/tissue interactions that maintain homeostatic balance and, on the other hand, dynamically respond to disturbances.
The lymphatic system presents an excellent model for studying short- and long-range tissue interactions in the context of adaptive immunity since lymphatics connect peripheral tissues to systemic circulation via tissue fluid draining and leukocyte trafficking. Further, the interactions between tissue compartments and cell types are dynamic and amenable to live-imaging approaches.
Recent years have revolutionized our understanding of the role of the lymphatic system in the control of adaptive immunity. To start an adaptive immune response, lymphatic vessel endothelium actively recruits antigen-presenting dendritic cells, to the lymphatic vessel lumen and, subsequently, guides them into the lymph nodes. In the lymph nodes, dendritic cells present the antigens and activate pathogen-specific T cells.
Our research group focuses on two key events that are essential for efficient antigen delivery into the lymph nodes. First, we explore how sprouting lymphangiogenesis is controlled to yield a well-structured functional lymphatic vessel network. Second, we investigate how lymphatic endothelium guides dendritic cell entry into the lymphatic system. To address these questions, we use in vivo models, tissue explants, primary cell co-culture assays, and state-of-the-art microscopy.
We expect that our studies form a basis for strategies to treat patients suffering from diseases in which inflammation is an essential component.
Our research group is intrigued by one of the most complex biological events: how migrating cells reach their destination, i.e. how cell migration guidance cues are generated and interpreted. Specifically, we investigate two key events of cell guidance: i) sprouting lymphangiogenesis and ii) dendritic cell entry into the lymphatic system.
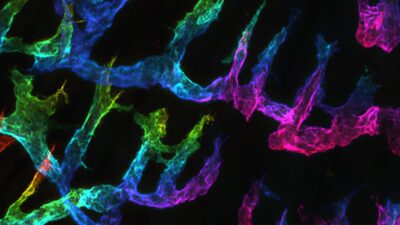
i) Lymphatic vessels form dense tree-like networks in various organs. The tree-top is formed by blind-ended lymphatic capillaries, which are sites of immune cell and tissue fluid entry. Although most of the capillaries are generated during development, lymphatic capillaries can also grow in length and caliber in adult tissues, e.g. in various conditions associated with inflammation. The density of the lymphatic capillaries correlates with the efficiency of tissue fluid drainage and inflammation resolution. Accordingly, experimentally induced lymphangiogenesis leads to enhanced T cell activation and enhances adaptive immunity. Thus, tools to enhance controlled lymphangiogenic sprouting, elongation, and subsequent maturation should allow enhanced adaptive immune responses.
We explore various opportunities to expand the lymphatic capillary networks in a controlled manner. Currently, we investigate the initial events of lymphangiogenic sprouting of mature lymphatic vessels. We want to know the cellular and molecular mechanisms that determine the site of lymphatic endothelial sprouting and allow lymphatic endothelial cells to escape the ordered structure of the endothelium and invade the interstitial tissue. In these studies, we use a combination of genetic and small-molecular approaches in in vivo-, tissue explant-, cell culture models, and computational approaches (in collaboration with Dr. Mehmet Can Ucar, University of Sheffield). These studies aim to identify overall principles governing the formation of lymphatic vessel networks.
ii) Lymphatic capillaries have been considered as passive conduits of tissue fluid. However, recent data indicates that lymphatic endothelial cells actively attract antigen-presenting dendritic cells into the lymphatic capillary lumen.
We have shown that lymphatic endothelial presentation of the chemokine is, in part, controlled at the level of exocytosis. We carried out the first characterization of chemokine CCL21 and CCL2 exocytosis routes and identified RAB6+ Golgi-derived vesicles and RAB3/27+ secretory granules as major lymphatic endothelial chemokine storage vesicles. Interestingly, RAB6 vesicles deliver the chemokines to lymphatic endothelial multicellular junctions, thus, defining the dendritic cell transmigration site. The RAB3/27+ secretory granules are located in the central body of the cell and may represent the conditional secretory vesicles, which we showed to be secreted “on-demand” upon dendritic cell – lymphatic endothelium interaction. These studies exemplify, how controlled presentation of guidance cues, in space and time, controls leukocyte migration. Our current aim is to identify molecular handles to manipulate the chemokine exocytosis in a secretory route-specific manner to dissect the contribution of each route in leukocyte guidance. In these studies, we use primary cell cultures, tissue explants, and in vivo models. We expect that these studies result in the identification of molecular handles that can be used for tuning the level of adaptive immunity.
Spatially targeted chemokine exocytosis guides transmigration at lymphatic endothelial multicellular junctions. Liaqat I., Hilska I., Jakobsson E., Saario M., Crivaro M., Peränen J., Vaahtomeri K. The EMBO Journal. 43(15):3141-3174, 2024
DLL4-Notch3-WNT5B axis mediates bi-directional pro-metastatic crosstalk between melanoma and lymphatic endothelial cells. Alve S., Gramolelli S., Jukonen J., Juteau S., Pink A., Manninen A., Monto E., Lackman MH., Carpén O., Karaman S., Saharinen P., Vaahtomeri K. and Ojala PM.
JCI insight, 9(1):e171821, 2023.
Self-organized and directed branching results in optimal coverage in developing dermal lymphatic networks. Can Ucar M., Hannezo E. , Tiilikainen E., Liaqat I., Jakobsson E., Nurmi, H., Vaahtomeri K. Nature Communications, 21;14(1):5878, 2023
Lymphangiogenesis requires Ang2/Tie/PI3K signaling for VEGFR3 cell surface expression. Korhonen E. A., Murtomäki A., Jha S.K., Anisimov A., Pink A., Zhang Y., Stritt S., Liaqat I., Stanczuk L., Alderfer L., Sun Z., Kapiainen E., Singh A., Sultan I., Lantta A., Leppänen V.M., Eklund L., He Y., Augustin H.G., Vaahtomeri K., Saharinen P., Mäkinen T., Alitalo K. 1 Aug 2022, J Clin Invest., 132(15):e155478.
Shape and function of interstitial chemokine CCL21 gradients are independent of heparan sulfates produced by lymphatic endothelium. Vaahtomeri K., Moussion C., Hauschild R., Sixt M. 25 Feb 2021, Front Immunol. 12:630002, 2021.
Locally triggered release of chemokine CCL21 promotes dendritic cell transmigration across lymphatic endothelia. Vaahtomeri K, Brown M, Hauschild R, De Vries I, Leithner AF, Mehling M, Kaufmann WA, Sixt M. 2 May 2017, Cell Reports, 19(5):902-909.
CCL21 promotes tissue egress of intralymphatic dendritic cells through afferent lymphatic vessels. Russo E., Teijeira A., Vaahtomeri K., Wilbrodt, AH., Bloch, JS., Nitschke, M., Santambrogio, L., Kerjaschki, D., Sixt, M., Halin, C.”, 23 February 2016, Cell Reports, 14(7):1723-1734.
Dendritic cells interpret Haptotactic Chemokine Gradients in a manner governed by signal-to-noise ratio and dependent on GRK6. Schwarz J, Bierbaum V, Vaahtomeri K, Hauschild R, Brown M, de Vries I, Leithner A, Reversat A, Merrin J, Tarrant T, Bollenbach T, Sixt M. 8 May 2017, Current Biology, 27(9):1314-1325.
Lymphatic exosomes promote dendritic cell migration along guidance cues. Brown M, Johnson LA, Leone DA, Majek P, Vaahtomeri K, Senfter D, Bukosza N, Schachner H, Asfour G, Langer B, Hauschild R, Parapatics K, Hong YK, Bennett KL, Kain R, Detmar M, Sixt M, Jackson DG, Kerjaschki D. 4 June 2018, Journal of cell biology, 217(6):2205-2221.
Current members
Kari Vaahtomeri, Principal investigator, Ph.D. Doc.
Inam Liaqat, Doctoral student
Maria Saario, Doctoral student
Jeremia Saari, Doctoral student
Mario Karam, Doctoral student
FORMER MEMBERS
Emmi Tiilikainen, completed M.Sc. thesis in the group
Sonja Granroth, completed M.Sc. thesis in the group
Emma Jakobsson, completed M.Sc. thesis in the group
Ida Hilska, completed M.Sc. thesis in the group