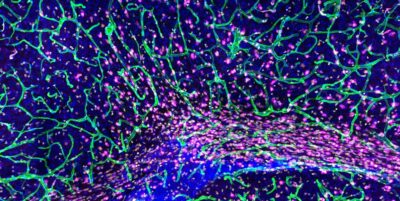
We study how blood and lymphatic vessels regulate central nervous system homeostasis and white matter integrity, focusing on vascular mechanisms that shape oligodendrocyte lineage cell function across development, aging, and repair.
Emilia Korhonen
AFFILIATED GROUP LEADER
PHD, ACADEMY RESEARCH FELLOW
CONTACT
EMILIA.Z.KORHONEN@HELSINKI.FI
Blood and lymphatic vessels, lined by endothelial cells, are essential for maintaining central nervous system (CNS) homeostasis. Endothelial cells regulate barrier properties, molecular exchange, metabolic balance, immune responses, and they release paracrine signals that influence surrounding cells, creating the controlled environment required for proper neural and glial function. Disturbance of these vascular systems can impair tissue homeostasis, limit repair capacity, and contribute to neurological diseases. Yet the mechanisms by which blood and lymphatic vessels support and communicate with CNS resident cells remain incompletely understood.
We investigate how blood and lymphatic vessels regulate oligodendrocyte lineage cells, the progenitor and myelinating cells responsible for maintaining white matter integrity, across development, homeostasis, aging, and injury. We examine how vascular cues shape OPC and oligodendrocyte function, and how disturbances in these pathways alter CNS homeostasis and repair. Our aim is to identify vascular mechanisms that support myelin maintenance and enhance white matter recovery in aging and neurological disorders.
Blood and lymphatic vasculatures are increasingly recognized as active regulators of CNS homeostasis. Endothelial cells influence glial populations through paracrine signaling, structural interactions and dynamic responses to homeostatic and inflammatory cues. Disturbances in blood or meningeal lymphatic vessel function, including those that arise with aging, are linked to impaired CNS stability and to neurological disorders involving inflammation, degeneration, or loss of myelin, yet the cellular and molecular mechanisms underlying these effects remain incompletely understood.
Oligodendrocyte lineage cells, including OPCs and myelinating oligodendrocytes, are essential for white matter integrity. Myelin produced by mature oligodendrocytes enables rapid signal conduction and provides metabolic support to axons, making the regulation of these lineage cells fundamental for CNS function. A growing body of work highlights a close association between the vasculature and oligodendrocyte lineage cells, forming the oligovascular niche, where vascular-derived cues influence OPC specification, migration, and lineage progression. However, how vascular dysfunction, inflammation and aging reshape this niche or contribute to impaired myelination and reduced repair remains largely unknown. These questions are central to understanding white matter vulnerability across development, aging, and disease.
Our group investigates how blood and lymphatic vessels regulate oligodendrocyte lineage dynamics and white matter integrity. We study how angiocrine signaling, vascular maturation, and lymphatic function shape the microenvironment that supports OPCs and oligodendrocytes, and how alterations in these processes affect myelin maintenance and CNS repair. By combining in vivo models with complementary in vitro systems, together with multi-omics and imaging approaches, we aim to uncover fundamental vascular mechanisms that govern white matter biology and to identify pathways that may be targeted to enhance myelin repair in aging and disease.
Korhonen EA, Murtomäki A, Jha SK, Anisimov A, Pink A, Zhang Y, Stritt S, Liaqat I, Stanczuk L, Alderfer L, Sun Z, Kapiainen E, Singh A, Sultan I, Lantta A, Leppänen VM, Eklund L, He Y, Augustin HG, Vaahtomeri K, Saharinen P, Mäkinen T, Alitalo K. Lymphangiogenesis requires Ang2/Tie/PI3K signaling for VEGFR3 cell-surface expression. J Clin Invest. 2022;132(15):e155478.
Leppänen VM*, Brouillard P*, Korhonen EA, Sipilä T, Kumar Jha S, Revencu N, Labarque V, Fastré E, Schlögel M, Ravoe M, Singer A, Luzzatto C, Angelone D, Giovanni Crichiutti G, D’Elia A, Kuurne J, Elamaa H, Koh GY, Saharinen P, Vikkula M#, Alitalo K#. Characterization of ANGPT2 mutations associated with primary lymphedema. Sci Transl Med. 2020;12:eaax8013. * # Equal contribution
Li Z*,Korhonen EA*, Merlini A, Strauss J, Wihuri E, Nurmi H, Antila S, Paech J, Deutsch U, Engelhardt B, Chintharlapalli S, Koh GY, Flügel A, Alitalo K. Angiopoietin-2 blockade ameliorates autoimmune neuroinflammation by inhibiting leukocyte recruitment into the CNS. J Clin Invest. 2020;130:1977-1990. * Equal contribution
Korhonen EA*, Lampinen A*, Giri H, Anisimov A, Kim M, Allen B, Fang S, D’Amico G, Sipilä TJ, Lohela M, Strandin T, Vaheri A, Ylä-Herttuala S, Koh GY, McDonald DM, Alitalo K#, Saharinen P#. Tie1 controls angiopoietin function in vascular remodeling and inflammation. J Clin Invest. 2016;126:3495-510. * # Equal contribution
D’Amico G, Korhonen EA, Anisimov A, Zarkada G, Holopainen T, Hägerling R, Kiefer F, Eklund L, Sormunen R, Elamaa H, Brekken RA, Adams RH, Koh GY, Saharinen P and Alitalo K. Tie1 deletion inhibits tumor growth and improves angiopoietin antagonist therapy. J Clin Invest. 2014;124:824-834.